|
News & Notices
Organizations
& People
Standing Committees
Divisions
Projects
Reports
Publications
Symposia
..Application for Sponsorship
..Visas
AMP
Links
of Interest
Search
the Site
Home
Page |
|
Pure Appl. Chem., Vol. 70, No. 11,
pp. 2128, 1998
The syntheses of lamellarins and isoindolobenzazepine
alkaloids*
Somsak Ruchirawat1,2,3, Thumnoon Mutarapat3,
Poolsak Sahakitpichan1, Vanida Bhavakul4,
and Chulabhorn Mahidol1
1. Chulabhorn Research Institute, Bangkok 10210, Thailand
2. Programme on Research and Development of Synthetic Drugs
3. Department of Chemistry, Mahidol University, Bangkok 10400, Thailand
4. Department of Chemistry, Faculty of Science, King Mongkut’s
University of Technology, Thonburi, Bangkok 10140, Thailand
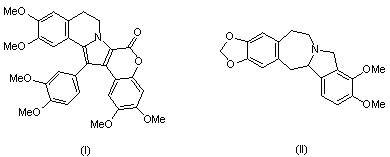
Abstract: Lamellarins are a group of marine natural products
which were isolated from the prosobranch mollusc Lemellaria sp
and the ascidians. Some of these lamellarins exhibit interesting
biological activities including cell division inhibition, cytotoxicity,
and immunological activity. The synthesis of lamellarin G trimethyl
ether (I) and other analogs will be presented.
Chilenine, isolated from the plants of Berberidaceae
by Shamma et al., is the first representative of the isoidolobenzazepine
alkaloids. Many synthetic routes to this class of alkaloids
will be briefly reviewed and our approach to the synthesis
of its analog, chilenamine (II) will be disclosed.
Download full text (6
pages) - PDF file (48KB)
* Invited lecture presented at the International
Conference on Bioversity and Bioresources: Conservation and
Utilization, 23-37 November 1997, Phuket, Thailand.
Page last modified 15 April 1999.
Copyright ©1997, 98, 99 International Union of Pure and Applied
Chemistry.
Questions or comments about IUPAC, please contact, the Secretariat.
Questions regarding the website, please contact Web Help.
|

