GUIDELINES
FOR DRAFTING IUPAC TECHNICAL REPORTS AND RECOMMENDATIONS (2007)
> 1.
Introduction
> 2. Form of the Manuscript
> 3. Definitions of Terms
> 4. Nomenclature
> 5. Quantities, Units and Symbols
> 6. Constructing a Terminology Entry in
a Glossary
> 7. Use of Terms from the IUPAC "Gold Book"
in Glossaires
> 8. Use of Italic and Roman Fonts for Symbols in
Scientific Text
> 9. Quantity Calculus
> 10. Percents and Per mils
> 11. Printing of Numbers
> 12. Abbreviations
> References
1. Introduction
These guidelines are offered by the Interdivisional
Committee on Terminology, Nomenclature and Symbols (ICTNS) to those
writing or planning to write documents for publication by IUPAC. ICTNS
has the task of ensuring that the contents of new recommendations concerned
with nomenclature, symbols, or definitions of terms are consistent with
previous such publications of the Union. Also, ICTNS scrutinizes IUPAC
technical reports of a more specialized nature for consistency of nomenclature
and symbols usage. Attention by authors to such matters at an early
stage of writing (or even planning) a document will speed substantially
the acceptance procedures for publication that ICTNS carries out on
behalf of the Union. Nothing is more annoying for the authors of a document,
who have worked hard to reach technical agreement on the contents with
their colleagues, than to be asked subsequently to make multiple changes
for consistency within IUPAC.
The situation with respect to ensuring consistency with earlier IUPAC
pronouncements has been much facilitated by the publication of three
reference works of general importance, namely the Compendium of Chemical
Terminology [the �Gold Book�; ref. 1], Principles
of Chemical Nomenclature - A Guide to IUPAC Recommendations [ref.
2], and Quantities, Units, and Symbols in Physical Chemistry [the
�Green Book�; ref. 3], to be discussed below.
In addition, authors of documents are advised strongly to send their
first full, or even partial, draft to their Division
representative on ICTNS, whose names are listed in the IUPAC Handbook,
for advice on whether or not they are adequately adhering to ICTNS�IUPAC
requirements. The Division representatives are asked to respond speedily,
with only general and helpful comments, and not to scrutinize the document
in a detailed manner at this stage. A full scrutiny will later be carried
out by ICTNS members on completed documents intended as Recommendations.
Technical Reports, which should not contain new material on symbols,
nomenclature, or definitions, are checked by the Officers of ICTNS before
submission for publication in Pure and Applied Chemistry.
Authors should indicate on the title page whether their manuscript
is intended to be a Recommendation or a Technical Report; their decision
on this matter will be subject to approval by the officers of ICTNS.
Time and effort will usually be saved if this ICTNS scrutiny is carried
out before the preparation of the final manuscript. In particular, the
title of each document should be informative to reflect well the contents
of the document and to permit its future retrieval from relevant databases
and indices through carefully chosen terms. If the author is not a native
English speaker, then it will frequently be profitable to consult an
English speaker for use of idioms, etc., before submission to ICTNS
via the Division President and the IUPAC Secretariat.
2. Form of the Manuscript
Technical Reports and Provisional Recommendations should be prepared
in the form of a high-quality manuscript, which is consistent with all
general recommendations of the Union on nomenclature and symbols. The
manuscript must include a abstract of approximately 200 words, along
with the name and address of the Task Group Member acting as document
editor, who will prepare the final draft for publication. Manuscripts
should preferably be submitted to the Secretariat in electronic form,
preferably using Micorsoft Word. Pages should be numbered. It is also
highly desirable that lines be numbered continuously through the manuscript
to facilitate location of text when comments are provided. (Pagination
is often changed in printing, whereas line numbering usually is unchanged
if the original margins are retained.) Particular attention should be
paid to the use of italic print for quantity symbols and upright print
for unit symbols, as described below.
3. Definitions of Terms
The format, style and content of documents containing Recommendations
or Technical Reports should follow the basic principles and examples
as described below in the section, 'Constructing a Terminology Entry
in a Glossary'. These principles and examples are sufficiently complete
that they can be used as guides in the construction of definitions of
terms. The specific requirements for the format of definitions are also
described below. If the document contains multiple definitions of terms,
as in a glossary, it is particularly helpful if these are numbered individually
and laid out in separate paragraphs for easy subsequent incorporation
into later editions of the �Gold Book �[ref. 1].
Definitions can be followed by separate explanatory notes and examples,
as required.
In compiling a glossary, the current edition of the 'Gold Book' should
be inspected in case a particular term has been defined previously within
IUPAC. If it has, but there is a need to repeat the definition, then
whenever possible the original wording should be retained. Whenever
necessary, the definition should be reformatted to conform with the
structure of a terminology entry given below. If it is considered essential
to change the wording, e.g., if the original is too technical for a
more general readership or if general terms need to be replaced by more
specific ones for a narrower readership, then the changes made should
be kept to a minimum and the justifying reasons given in a footnote,
together with a reference to the original 'Gold Book' entry.
In certain areas, and particularly in metrology, it is important for
IUPAC to retain consistency with definitions given by �higher authorities�
such as in the International Vocabulary of Basic and General Terms in
Metrology [VIM; ref. 45] and the International
Organization for Standardization [ISO; refs. 46 and
48]. The definitions given by these bodies are agreed upon after
much international consultation, and they have official status in connection
with trade and/or legal usage. Again, any required rewordings for different
usage should be specified and justified.
An IUPAC document should always fully list other sources that have
been consulted for purposes of consistency. General references to the
others sources consulted should be made within the document; more specific
references need be given only when changes to the original are made
as discussed above. The citation style for references should follow
that given in PAC
Instructions for Authors [www.iupac.org/publications/authors/instructions.html].
References to other IUPAC documents should include the full title of
the document.
Abbreviations, initialisms, and acronyms tend to be understood only
by limited groups of practitioners. They should be used sparingly and
always defined once in any document [the �Green Book�; ref.
3, section 9; also ref. 5]. In addition,each
document should list separately all the abbreviations, initialisms,
and acronyms in alphabetical order, with their full definitions, preferably
in a table just ahead of the list of references. For the same reason,
general laboratory slang, pertaining to a particular field, should not
be used in written reports, as this will often not be understood by
readers versed in related areas of chemistry, who nevertheless wish
to gain an understanding of the new field.
4. Nomenclature
Nomenclature in all areas of chemistry is continuously under development
within IUPAC, and the pages of Pure and Applied Chemistry should
be consulted for recent Recommendations. Several important book-form
compendia exist for Inorganic Chemistry [the �Red Book�; ref.
6 and �Red Book II�; ref. 7], Organic Chemistry
[the �Blue Book�and associated �Guide�and �Glossary�; refs.
8�11], Macromolecular Chemistry [the �Purple Book�; ref.13
superseded in part by refs. 14-29], and Analytical
Chemistry [the �Orange Book�and �Sampling Nomenclature Recommendations�;
refs. 38 and 39]. Copies of these will be sent
to Division Officers within the relevant Division. Recommendations and
a compendium of Biochemical Nomenclature [refs. 42
and 43 ] have been compiled under the aegis of the IUPAC�IUBMB
Joint Commission on Biochemical Nomenclature.
In the preparation of nomenclature documents it is also helpful, as
for definitions, if individual rules are given in numbered paragraphs
together with explanatory notes and examples, as required.
In IUPAC Recommendations that are not concerned with new nomenclature,
names of chemical substances should be in accordance with published
IUPAC rules [refs. 2, 6-29 and
42]. In those cases where these rules allow several
names for the same molecular entity, ICTNS may ask authors, during the
review process, to use a different alternative, according to the aims
and context of the Recommendation. Instead of IUPAC names, certain common
names are acceptable in the following cases: INNs (International Nonproprietary
Names) are allowed for pharmaceutical substances [ref.
30]. For agricultural chemicals including pesticides, ISO names
can also be used [refs. 31-37]. For recently discovered
natural products with complex structures, where systematic names would
be too unwieldy and no retained IUPAC names exist as yet, names derived
from those of the biological materials in which they occur are acceptable.
Biochemical names from IUPAC and IUBMB are also allowed [ref.
42, see also ref. 43]. In IUPAC Technical Reports,
the same rule holds. In Technical Reports, very often a name is quoted
from an existing publication. This name should be retained, with an
IUPAC or another internationally approved name following in square brackets.
5. Quantities, Units and Symbols
The 'Green Book' [ref. 3] provides an up-to-date
account of IUPAC requirements in these areas. An abbreviated list is
also available [ref. 4]. During the writing of the
'Green Book', careful harmonization was made with analogous recommendations
from ISO and from the International Union of Pure and Applied Physics
(IUPAP).
Certain rules in the 'Green Book' must be strictly adhered to, namely,
the italic letters for symbols for physical quantities and roman letters
for units (section 1.6), and the instructions for the printing of numbers
and mathematical symbols (section 4.1). These points are highlighted
below.
When a document includes recommendations for a number of new quantities
and symbols, as may often happen in dealing with a specialized area
of chemistry, it will generally be helpful to provide a summary table
of all quantities included in the document, similar to the tables in
Chapter 2 of the 'Green Book', with the headings: Name, Symbol, Brief
defining equation, SI Unit, Notes. Tables of this kind allow the reader
to recover important information quickly when referring to a document
for the second or third time, and allow easy comparison between different
documents.
The specific use of quantities and units in Clinical Chemistry is discussed
in refs. 40 and 41.
6. Constructing a Terminology Entry in
a Glossary
A specific format is required for entries in a glossary, or for definitions
of terms in general. Adherence to this format will facilitate its eventual
incorporation in the 'Gold Book', and will also help to ensure that
the term is explained accurately, logically and completely.
The general outline of the structure of a terminology entry is as follows.
Annotations (which are not part of the terminology entry) are denoted
as numbers in parentheses and are explained at the end of the section.
Note carefully:
(a) use of punctuation and different typefaces, both of which should
be as used in the outline;
(b) the first five entries, as appropriate, are placed on one line,
or continued on the following line, if necessary.
entry number (1), preferred term (2), (acronym)
(3), word class (4), symbol (5)
synonym (6)
deprecated term (6)
obsolete term (6)
superseded term (6)
definition of term (7)....cross-referenced term (8)....reference (9)
Note 1: (10)
Note 2: (10)
......
Example: (11)
Related term(s): (12)
References: (13)
Explanation of Annotations
(1) Entry numbers in a glossary are assigned successively to terms
arranged in alphabetical order. If the glossary contains more than
one section, terms are arranged in alphabetical order within sections,
but the numerical order is continuous throughout the document. The
main use of entry numbers is in editing, in review, and to facilitate
transfer to the 'Gold Book'.
(2) (a) Use lower-case bold type, except when a proper name is part
of the term.
(2) (b) For entries in inverted order, e.g., tracer, generally
labelled, add on the following line 'See (entry in direct order)',
e.g., 'See generally labelled tracer.'
(2) (c) For homographic terms (those with the same spelling but different
meanings), add an explanatory adjective in parentheses, and treat
each term as a separate entry; e.g., 'configuration (electronic)',
'configuration (stereochemical)'.
(2) (d) Cross-references are listed alphabetically along with other
terms.
(3) Optional; printed in the same typeface as the preferred term.
(4) Word class (or part of speech) is necessary only if the term
can be used as more than one part of speech, in which case the particular
part of speech is identified as an italicized abbreviation; e.g.,
n. (noun), v. (verb), adj. (adjective), adv.
(adverb).
(5) Required if appropriate; printed in the typeface as described
below or in the 'Green Book' [ref. 3]. If used, the symbol should
follow recommendations in the 'Green Book'.
(6) Add if necessary. Deprecated terms include those that
have been in common use, but are not compatible with IUPAC recommendations;
e.g., 'number of moles of X' instead of the recommended 'amount of
X'. Obsolete terms include those that may be encountered in
older literature, but are no longer in use; e.g., 'gram-molecular
weight'. Superseded terms include those that can still be found
in the literature, but can be expressed more logically in newer terms;
e.g., 'specific gravity' instead of the more logical 'relative density'.
The distinction between 'obsolete' and 'superseded' terms is clearly
somewhat arbitrary. It is not expected that many terms will appear
in glossaries under these classifications.
(7) For new definitions (those that have not appeared in previous
IUPAC Recommendations), start the entry with a capital letter but
do not use an article. The definition should be in the same word class
(noun, adjective, verb, adverb) as the term itself.
In defining terms involving a ratio of two quantities, do not use
"ratio of" or "quotient of". The phrase "divided
by" is recommended. Phrases like "x per unit y" should
never be used, as they confuse physical quantities and units, and
can be ambiguous.
(8) Cross-referenced terms are in italics.
(9) References can be added here as numbers in square brackets, e.g.,
[3]. If desired, they can be placed in introductory text. If a definition
from the 'Gold Book' is included as part of a glossary, then specific
reference to that publication should be made for completeness and
as a signal to the compilers of the 'Gold Book'. If the 'Gold Book'
definition is used with no changes, then no references other than
to the 'Gold Book' are necessary. If, however, changes to the definition
are recommended, additional supporting references should be added.
Reference to the 'Gold Book' is preferably in the form: 'Gold Book
online, 1997 entry', 'Gold Book online, 2003 entry', etc.
(10) Explanatory notes can be added here, as many as necessary.
(11) An example of use of the term can be given here.
(12) Related terms can be added here.
(13) The list of references should be added here. The format should
follow that used in current issues of Pure and Applied Chemistry.
Specific examples > see pdf version (61KB)
These examples are taken from the 'Gold Book 1997' [ref.
1a].
7. Use of Terms from the IUPAC "Gold Book"
in Glossaries
7.1. General Instructions. Entries from the "Gold Book"
should be reproduced in new Recommendations exactly, unless the conditions
of instruction 2 apply. Authors should remember that any new definitions
of terms will appear eventually in the "Gold Book", and the
compilers of the "Gold Book" should not have to make a decision
between existing and new definitions.
There are two minor but universal exceptions:
(1) In the "Gold Book", entries have frequently combined
a definition with a commentary on this definition. According to the
previous sections of these Guidelines, commentaries or further explanations
of the definition should be placed in notes, so that the definition
and comments on it are clearly separated. This is the recommended
procedure, even though there are no plans for a systematic revision
of all definitions in the "Gold Book".
(2) Starting an entry with a definite or indefinite article is not
acceptable, according to the Guidelines.
Example:
asymmetric
Lacking all symmetry elements (other than the trivial one of
a one-fold axis of symmetry), i.e. belonging to the symmetry point group
C1. The term has been used loosely (and incorrectly)
to describe the absence of a rotation-reflection axis (alternating axis)
in a molecule, i.e. as meaning chiral, and this usage persists in the
traditional terms asymmetric carbon atom, asymmetric synthesis, asymmetric
induction, etc.
In this entry from the "Gold Book", the first sentence is
clearly the definition, while the second sentence is a commentary on
use of the term. Therefore, the second sentence should be placed in
a Note when used in new Recommendations.
7.2. Modification of Entries. Entries should be modified only
after careful consideration. Continual modification of entries is counterproductive
to maintaining the Gold Book as an authoritative source. Entries may
be modified in the following circumstances:
(1) The entry contains one or more errors. The author should correct
the error(s), and add, at the end of the entry, "Corrected from
[x].", where [x] is the reference number for the "Gold Book".
Reviewers will be able to question this correction.
(2) The author considers that an entry is, for example, not clearly
written, superseded by newer developments, not sufficiently complete
for the present Recommendations, etc. The author can propose a new
definition or new Notes, or both, but must be ready to defend the
changes to reviewers. At the end of the entry, the words: "Modified
from [x]." should be added, along with any supporting references.
References should be at the end of the entry, not within the text.
Changing an existing "Gold Book" entry implies that the
entry becomes longer, through either the definition itself or notes.
Longer entries in the form of explanatory notes usually make the entry
more useful to the reader. It is difficult to conceive that a shorter
entry will be acceptable.
8. Use of Italic and Roman Fonts for Symbols in
Scientific Text
> reproduced from original summary by I.M. Mills and W.V.
Metanomski, December 1999. [ref. 49]
Scientific manuscripts frequently fail to follow the accepted conventions
concerning the use of italic and roman fonts for symbols. An italic
font is generally used for emphasis in running text, but it has a quite
specific meaning when used for symbols in scientific text and equations.
The following summary is intended to help in the correct use of italic
in preparing manuscript material.
8.1. The general rules concerning the use of an italic (sloping) font
or a roman (upright) font are presented in the IUPAC Green Book [ref.
3] on p. 5 and 6, and also p. 83 to 86 in relation to mathematical
symbols and operators (see also p. 75, 76, and 93). These rules are
also presented in the International Standards ISO 31 and ISO 1000,*
and in the SI Brochure [ref. 44].
* The ISO Standards Handbook, Quantities and Units,
ISO, Geneva, 1993.
8.2. The overall rule is that symbols representing physical quantities
(or variables) are italic, but symbols representing units, or labels,
are roman. Sometimes there may seem to be doubt as to whether a symbol
represents a quantity or has some other meaning (such as a label): a
good general rule is that quantities, or variables, can be given a value,
but labels cannot. Vectors and matrices are usually denoted using a
bold-face (heavy) font, but they should still be italic since they are
still quantities.
Example: The mass of my pen m = 24 g = 0.024 kg.
The electric field strength E has components Ex,
Ey, and Ez.
The Planck constant h = 6.626 068 76 (52) x
10-34 J s.
8.3. The above rule applies equally to letter symbols from both the
Greek and the Latin alphabet, although authors often appear to resist
putting Greek letters into italic.
Example: when the symbol m is
used to denote a physical quantity (such as mass or reduced mass)
it should be italic, but when it is used in a unit such as the microgram,
mg, or
when it is used as the symbol for the muon, m
(see 5 below), it should be roman.
8.4. Numbers, and labels, are generally roman (upright), since they
are not physical quantities.
Example: The ground and first excited electronic states
of the CH2 molecule are denoted
. . . 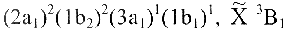
and
. . . 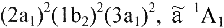
respectively.
The p-electron configuration and symmetry
of the benzene molecule in its ground state are denoted:
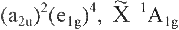
Note that all these symbols are labels and are roman.
8.5. Symbols for elements in the periodic table should be roman, since
they are not physical quantities. Similarly the symbols used to represent
elementary particles are always roman. (See, however, paragraph 9 below
for the use of italic font in chemical-compound names.)
Examples: H, He, Li, Be, B, C, N, O, F, Ne, . . . for atoms;
e for the electron, p for the proton, n for the neutron, m for the muon, a for the alpha
particle, etc.
8.6. Symbols for physical quantities are single letters of the Latin
or Greek alphabet. Exceptionally two letters are used for certain dimensionless
quantities, such as the Reynolds number, Re. However the symbols
are frequently supplemented with subscripts or information in brackets
to further specify the quantity. Further symbols used in this way are
either italic or roman depending on whether they represent physical
quantities or labels.
Examples: H denotes enthalpy, but Hm
denotes molar enthalpy (m is a mnemonic label for molar, and is
therefore roman).
Cp and CV
denote the heat capacity at constant pressure
p and volume V, respectively; but Cp,m
and CV,m
denote the molar heat capacity at constant p and V,
respectively (note the roman m but italic p and V).
The chemical potential of argon might be denoted mAr
or m(Ar), but the chemical potential
of the ith component in a mixture would be denoted mi
, where the i is italic because it is a variable index.
8.7. Symbols for mathematical operators are always roman. This applies
to the symbol D for
a difference, d for a small difference, d
for an infinitesimal difference (in calculus), and to capital S
and P for summation and product signs. The symbols p,
e (base of natural logarithms), i (square root of minus one), etc. are
always roman, as are the symbols for named functions such as log (lg,
ln or lb), exp, sin, cos, tan, erf, div, grad, curl or rot
(the operator curl or rot, and the corresponding symbol -see
pdf version of this symbol- may be printed boldface since it represents a vector). Some
of these symbols are also sometimes used to represent physical quantities:
then of course they should be italic, to distinguish them from the corresponding
mathematical operator.
Examples: DH = H(final) - H(initial); (dp/dt)
used for the rate of change of pressure; dt
used to denote a small time interval. But for a damped
linear oscillator the amplitude F as a function of time t
might be expressed by the equation: F = F0
exp(-dt) sin(wt)
where d
is the decay coefficient (SI unit: Np/s) and w
is the angular frequency (SI unit: rad/s). Note the use of roman
d for
the operator in a small time interval dt,
but italic d for the decay coefficient
in the product dt. Note
that the products dt and
wt are both dimensionless,
but are described as having the unit neper (Np = 1) and radian (rad
= 1), respectively.
8.8. Symbols for the fundamental physical constants are always regarded
as quantities (even though they are not quite variables!) and they should
accordingly always be italic. Sometimes the fundamental physical constants
are used as though they were units, but they are still given italic
symbols. However the electronvolt, eV, and the unified atomic mass unit,
u, have been recognized as units by the Consultative Committee on Units
of the BIPM and they are accordingly given roman symbols.
Examples: c0 for
the speed of light in vacuum, me
for the electron rest mass, h for
the Planck constant, NA
or L for the Avogadro constant, e for the elementary
charge, a0 for the Bohr
radius, etc. The electronvolt eV = e x
V = 1.602 176 462 (63) x
10-19 J, the symbol eV is roman.
8.9. (revised by ICTNS in May 2007)
Greek letters are used in some cases for certain purposes in systematic
organic, inorganic, polymer, and biochemical nomenclature. These should
be in roman (upright) type. They designate e. g. the position of substituents,
double bonds, ligating-atom attachment and bridging mode in coordination
compounds, end groups in structure-based names for polymers, and configuration
in carbohydrates and natural products.
Letter symbols for elements are italic when used in names indicating
attachments to heteroatoms, e.g., O-, N-, S-, and
P-. The italic element symbol H denotes indicated or added
hydrogen. See references [ref. 2] and [ref.
9].
> Examples
[open jpg]
9. Quantity Calculus
> See original
summary [ref. 50], by T. Cvitas, February 2002
10. Percents and Per Mils, Parts Per Million
> See original
summary [ref. 50 and 51], by T. Cvitas, February 2002
11. Printing of Numbers
Numbers shall be printed in roman (upright) and lightface fonts [ref.
46, ISO 31]. Hence, numerical values of physical quantities (and
the symbols for their units) shall be printed in roman, lightface fonts,
irrespective of the type used in the rest of the text.
The decimal sign for IUPAC publications in English shall be a point
on the line. However, in languages other than English, the comma on
the line is often used as the decimal sign, as, for example, in French,
German, and Russian.
If the magnitude of the number is less than one, the decimal sign shall
be preceded by a zero. For numbers with many digits, the digits may
be separated in groups of three, counting from the decimal sign toward
the left and the right. The groups should be separated by a thin space
(half space), and never by a comma or a point, or by any other means.
(This space is best produced as a non-breaking space of constant width,
which also prevents splitting of numbers on line breaks. In MS Word
for Windows, type ctrl-shift-space; in Mac OS, use command-space.)
When a number with only four digits before or after the decimal sign
is given in running text the small space may be left out in order not
to isolate a single digit. However, both recommendations about grouping
digits into groups of three and not isolating a single digit are to
some extent at the discretion of the author. There may be occasions
when it is desirable to leave no spaces at all, for example, in numbers
to be printed or read by a computer. There shall never be a space in
a year written with four digits, for example, 1935 (not 1 935). Similarly,
there may be occasions when it is desirable to isolate a single digit,
for example, in formatting a column (in a table) of numbers which have
varying number of digits before or after the decimal sign.
When uncertainties in the final one or two digits of a numerical value
are given as one or two digits in parentheses, there can be either no
space or a thin space (half space) between the final digit of the numerical
value and the left parenthesis. See also ref. 47.
Examples:
Numbers in a running text: 3.1416
or 3.141 6
Numbers in a column:
01
000.234 5
21 110.216 48
000500.123 3
12. Abbreviations
BIPM - International Bureau of Weights and Measures (Bureau international
des poids et mesures)
ICTNS - Interdivisional Committee on Terminology, Nomenclature and
Symbols (of IUPAC)
IEC - International Electrotechnical Commission
IFCC -International Federation of Clinical Chemistry
ISO - International Organization for Standardization
IUBMB - International Union of Biochemistry and Molecular Biology
IUCr - International Union of Crystallography IUNS International
Union of Nutritional Sciences
IUNS - International Union of Nutritional Sciences
IUPAC - International Union of Pure and Applied Chemistry
IUPAP - International Union of Pure and Applied Physics
IUPHAR - International Union of Pharmacology
OIML - International Organization of Legal Metrology (Organisation
Internationale de Métrologie Légale)
WHO - World Health Organization
References
>
IUPAC
Nomenclature Books Series
General and Physical Chemistry
1. (a) IUPAC. Compendium of Chemical
Terminology, 2nd ed. (the "Gold Book"). Compiled by
A.D. McNaught and A. Wilkinson, Blackwell Scientific Publications,
Oxford, UK (1997). (b) XML on-line corrected version: http://goldbook.iupac.org
(2006- ) created by M. Nic, J. Jirat, and B. Kosata; updates compiled
by A.D. Jenkins.
2. G.J. Leigh, H.A. Favre, and W.V. Metanomski;
G.J. Leigh (Ed.), Principles of Chemical Nomenclature - A Guide
to IUPAC Recommendations. Blackwell Science, Oxford, UK (1998).
> online
version
3. IUPAC Physical and Biophysical Chemistry
Division, Quantities,
Units and Symbols in Physical Chemistry. (The IUPAC 'Green
Book'), 3rd edition. Prepared for publication by E.R. Cohen, T. Cvitaš,
J.G. Frey, B. Holmström, K. Kuchitsu, R. Marquardt, I. Mills,
F. Pavese, M. Quack, J. Stohner, H.L. Strauss, M. Takami, and A.J.
Thor. RSC Publishing, Cambridge 2007.
4. IUPAC Commission on Physicochemical
Symbols, Quantities and Units, Abbreviated List of Quantities,
Units and Symbols in Physical Chemistry. Prepared for publication
by K.H. Homann. Blackwell Scientific Publications, Ltd., Oxford, UK
(1993).
> online version
5. IUPAC Interdivisional Committee on Nomenclature
and Symbols, Use of Abbreviations in the Chemical Literature: IUPAC
Recommendations 1979. Prepared for publication by D.R. Lide, Jr. Pure
Appl. Chem. 52, 2229-2232 (1980).
> online version
Inorganic Chemistry
6. IUPAC Division of Chemical Nomenclature
and Structure Representation, Nomenclature
of Inorganic Chemistry - IUPAC Recommendations 2005. Prepared
for publication by N.G. Connelly, T. Damhus, R.M. Hartshorn and A.T.
Hutton. RSC Publishing, London (2005).
7. IUPAC Commission on the Nomenclature
of Inorganic Chemistry, Nomenclature of Inorganic Chemistry II,
Recommendations 2000. (The IUPAC 'Red Book II'). J.A. McCleverty
and N.G. Connelly (Eds.). Royal Society of Chemistry, Cambridge, UK
(2000).
Superseded in part by ref. 6.
Organic Chemistry
8. IUPAC Commission on the Nomenclature
of Organic Chemistry, Nomenclature of Organic Chemistry. (The
IUPAC 'Blue Book'). Sections A, B, C, D, E, F, and H. Prepared for
publication by J. Rigaudy and S.P. Klesneym, Pergamon Press, Oxford,
UK (1979).
The placement of locants used in these Recommendations is superseded
by rule R - 0.1.2 of ref. 9. - Sections F and H are entirely superseded
by Revised Sections F and H (see ref. 12)
9. IUPAC Commission on the Nomenclature
of Organic Chemistry, A Guide to IUPAC Nomenclature of Organic
Compounds. R. Panico, W.H. Powell, J.-C. Richer (Eds.). Blackwell
Scientific Publications, Ltd., Oxford, UK (1993).
Supersedes in part ref. 8.
10. IUPAC Commission on the Nomenclature
of Organic Chemistry, Corrections to 'A Guide to IUPAC Nomenclature
of Organic Compounds'. IUPAC Recommendations 1993. Prepared by H.A.
Favre, K.-H. Hellwich, G.P. Moss, W.H. Powell, J.G. Traynham. Pure
Appl. Chem. 71, 1327-1330 (1999).
11. IUPAC Commission on the Nomenclature
of Organic Chemistry and Commission on Physical Organic Chemistry,
Glossary of Class Names of Organic Compounds and Reactive Intermediates
Based on Structure. IUPAC Recommendations 1995. Prepared for publication
by G.P. Moss, P.A.S. Smith, D. Tavernier. Pure Appl. Chem.
67, 1307-1375 (1995).
12. IUPAC organic nomenclature recommendations published since
1993 are accessible from http://www.chem.qmul.ac.uk/iupac/.
Revised Sections F and H supersede entirely the same sections in ref.
7.
Polymer Chemistry
13. IUPAC Commission on Macromolecular
Nomenclature, Compendium of Macromolecular Nomenclature. (The
IUPAC 'Purple Book'). Prepared for publication by W.V. Metanomski.
Blackwell Scientific Publications, Ltd., Oxford, UK (1991).
> online
version
Superseded in part by refs. 14 - 29.
14. IUPAC Commission on Macromolecular
Nomenclature, Glossary of Basic Terms in Polymer Science. IUPAC Recommendations
1996. Prepared for publication by A.D. Jenkins, P. Kratochvíl,
R.F.T. Stepto, U. W. Suter. Pure Appl. Chem. 68, 2287-2311
(1996).
15. IUPAC Commission on Macromolecular Nomenclature, Graphic
representations (chemical formulae) of macromolecules, IUPAC Recommendations
1994. Prepared by a Working Group consisting of R.E. Bareiss, J. Kahovec
and P. Kratochvíl, Pure Appl. Chem. 66, 2469-2482 (1994).
16. IUPAC Commission on Macromolecular Nomenclature, Basic
classification and definitions of polymerization reactions, IUPAC
Recommendations 1994. Prepared by a Working Group consisting of I.
Mita, R.F.T. Stepto and U.W. Suter, Pure Appl. Chem. 66, 2483-2486
(1994).
17. IUPAC Commission on Macromolecular Nomenclature, Definition
of terms relating to degradation, aging, and related chemical transformations
of polymers, IUPAC Recommendations 1996. Prepared by a Working Group
consisting of K. Hatada, R.B. Fox, J. Kahovec, E. Maréchal,
I. Mita and V.P. Shibaev, Pure Appl. Chem. 68, 2313-2323 (1996).
18. IUPAC Commission on Macromolecular Nomenclature, Definitions
of terms relating to the non-ultimate mechanical properties of polymers,
IUPAC Recommendations 1998. Prepared by a Working Group consisting
of A. Kaye, R.F.T. Stepto, W.J. Work , J.V. Alemán and A.Y.
Malkin, Pure Appl. Chem. 70, 701-754 (1998).
19. IUPAC Commission on Macromolecular Nomenclature, Definitions
of basic terms relating to low-molar-mass and polymer liquid crystals,
IUPAC Recommendations 2001. Prepared for publication by M. Barón,
Pure Appl. Chem. 73, 845-895 (2001).
20. IUPAC Commission on Macromolecular Nomenclature, Definitions
of basic terms relating to polymer liquid crystals, IUPAC Recommendations
2001. Prepared for publication by M. Barón and R.F.T. Stepto,
Pure Appl. Chem. 74, 493-509 (2002).
21. IUPAC Commission on Macromolecular Nomenclature, Nomenclature
of regular double-strand (ladder and spiro) organic polymers, IUPAC
Recommendations 1993. Prepared by a Working Group consisting of W.V.
Metanomski, R.E. Bareiss, J. Kahovec, K.L. Loening, L. Shi and V.P.
Shibaev, Pure Appl. Chem. 65, 1561-1580 (1993).
22. IUPAC Commission on Macromolecular Nomenclature, Structure-based
nomenclature for irregular single-strand organic polymers, IUPAC Recommendations
1994. Prepared by a Working Group consisting of R.B. Fox, N.M. Bikales,
K. Hatada and J. Kahovec, Pure Appl. Chem. 66, 873-889 (1994).
23. IUPAC Commission on Macromolecular Nomenclature, Nomenclature
of regular single-strand organic polymers, IUPAC Recommendations 2002.
Prepared by a Working Group consisting of J. Kahovec, R.B. Fox, and
K. Hatada, Pure Appl. Chem. 74, 1921-1956 (2002).
24. IUPAC Commission on Macromolecular Nomenclature, Source-based
nomenclature for non-linear macromolecules and macromolecular assemblies,
IUPAC Recommendations 1997. Prepared by a Working Group consisting
of J. Kahovec, P. Kratochvíl, A.D. Jenkins, I. Mita, I.M. Papisov,
L.H. Sperling and R.F.T. Stepto, Pure Appl. Chem. 69, 2511-2521
(1997).
25. IUPAC Commission on Macromolecular Nomenclature, Definitions
relating to stereochemically asymmetric polymerizations, IUPAC Recommendations
2001. Prepared by a Working Group consisting of K. Hatada, J. Kahovec,
M. Barón, K. Horie, T. Kitayama, P. Kubisa, G.P. Moss, R.F.T.
Stepto, and E.S. Wilks, Pure Appl. Chem. 74, 915-922 (2002).
26. IUPAC Commission on Macromolecular Nomenclature, Generic
source-based nomenclature for polymers, IUPAC Recommendations 2001.
Prepared for publication by E. Maréchal and E.S. Wilks, Pure
Appl. Chem. 73, 1511-1519 (2001). Errata, Pure Appl. Chem.
74, 2019 (2002).
27. IUPAC Commission on Macromolecular Nomenclature, Definitions
of terms related to polymer blends, composites, and multiphase polymeric
materials, IUPAC Recommendations 2004. Prepared by a Working Group
consisting of W.J. Work, K. Horie, M. Hess, and R.F.T. Stepto, Pure
Appl. Chem. 76, 1985-2007 (2004).
28. IUPAC Commission on Macromolecular Nomenclature, Definitions
of terms relating to reactions of polymers and to functional polymeric
materials, IUPAC Recommendations 2003. Prepared by a Working Group
consisting of K. Horie, M. Báron, R. B. Fox, J. He, M. Hess,
J. Kahovec, T. Kitayama, P. Kubisa, E. Maréchal, W. Mormann,
R.F.T. Stepto, D. Tabak, J. Vohlídal, E.S. Wilks, and W. J.
Work, Pure Appl. Chem. 76, 889-906 (2004).
29. Regular Single-Strand and Quasi-Single Strand Inorganic
and Coordination Polymers, Chapter II-7 of reference 7.
Common Names
30. WHO World Health Organization, International
Nonproprietary Names (INN) for Pharmaceutical Substances, List of
Proposed INN and List 1 - 84 of Recommended INN. Cumulative List 10.
Geneva 2001.
31. ISO International Organization for
Standardization, Pesticides and other Agrochemicals - Common names,
ISO 1750, 1981.
32. ISO International Organization for Standardization, Pesticides
and other Agrochemicals - Common names, ISO 1750, 1981/Add.1, 1983.
33. ISO International Organization for Standardization, Pesticides
and other Agrochemicals - Common names, ISO 1750, 1981/Add. 2, 1983.
34. ISO International Organization for Standardization, Pesticides
and other Agrochemicals - Common names, ISO 1750, 1981/Amd. 1, 1982.
35. ISO International Organization for Standardization, Pesticides
and other Agrochemicals - Common names, ISO 1750, 1981/Amd. 2, 1999.
36. ISO International Organization for Standardization, Pesticides
and other Agrochemicals - Common names, ISO 1750, 1981/Amd. 3, 2001.
37. ISO International Organization for Standardization, Pesticides
not considered to require common names, ISO 765, 1976.
Analytical Chemistry
38. IUPAC Analytical Chemistry Division,
Compendium of Analytical Nomenclature (The IUPAC 'Orange Book'),
3rd edition. Prepared for publication by J. Inczédy, T. Lengyel,
A.M. Ure. Blackwell Science, Ltd., Oxford, UK (1998).
> online
version
39. IUPAC Analytical Chemistry Division,
Nomenclature for Sampling in Analytical Chemistry. IUPAC Recommendations
1990. Prepared for publication by W. Horwitz. Pure Appl. Chem.
62, 1193-1208 (1990).
Clinical Chemistry
40. IUPAC Commission on Quantities and
Units in Clinical Chemistry and IFCC Commission on Quantities and
Units, Compendium of Terminology and Nomenclature of Properties
in Clinical Laboratory Sciences. Recommendations 1995 (the IUPAC/IFCC
'Silver Book'). J.C. Rigg, S.S. Brown, R. Dybkaer, H. Olesen (Eds.).
Blackwell Science, Ltd., Oxford, UK (1995).
41. Glossary of Terms in Quantities and
Units in Clinical Chemistry. IUPAC-IFCC Recommendations 1996. Prepared
for publication by H.P. Lehmann, X. Fuentes-Arderiu, L. F. Bertello.
Pure Appl. Chem. 68, 957-1000 (1996).
Biochemistry
42. Recommendations on Biochemical Nomenclature
by IUPAC and IUBMB (International Union of Biochemistry and Molecular
Biology) from JCBN (Joint Commission on Biochemical Nomenclature)
are accessible from www.chem.qmul.ac.uk/iupac/,
as well as IUBMB Recommendations.
43. Committee of editors of Biochemical
Journals, Biochemical Nomenclature and Related Documents-A Compendium.
(The IUBMB 'White Book'); 2nd edition. C. Liébecq (Ed.). Portland
Press, London, UK (1992).
Quantities, Units and General Metrology
44. Bureau International des Poids et
Mesures (BIPM), Le Système International d'Unit�s (with English
translation); 8th edition. Sèvres, France (2006).
> online at www.bipm.org/en/si/
45. BIPM, IEC, IFCC, ISO, IUPAC, IUPAP,
OIML, International Vocabulary of Basic and General Terms in Metrology
(VIM). ISO, Geneva, Switzerland (1993).
46. Quantities and Units -ISO Standards
Handbook. Contains ISO 1000 and ISO 31/0 to 13 ISO, Geneva, Switzerland
(1993).
Available from booksellers (ISBN 92-67-10185-4) and from national
standards institutions.
47. BIPM, IEC, IFCC, ISO, IUPAC, IUPAP,
OIML, Guide to the Expression of Uncertainty in Measurement.ISO, Geneva,
Switzerland (1993). Available only from national standards institutions.
Statistics
48. Statistical Methods -ISO Standards
Handbook 3. Contains especially ISO 3534 Statistics- Vocabulary
and Symbols. ISO, Geneva, Switzerland (1989). Available only from
national standards institutions.
Bibliography and Editing
49. I. M. Mills and W. V. Metanomski,
Report from IDCNS, December, 1999.
> online
version
50. T. Cvita�, Report from ICTNS, February
2002
> online
version
51. T. Cvitaš, Quantities describing
compositions of mixtures. Metrologia 33, 35-39 (1996).
See also >
Notes for Contributors to PAC

