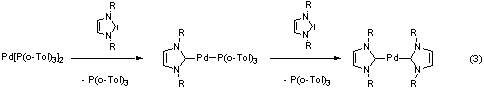|
News
& Notices
.. Prize
for Young Chemists
.... 2000 winners
....
2001 winners
Organizations & People
Standing
Committees
Divisions
Projects
Reports
Publications
Symposia
AMP
Links
of Interest
Search
the Site
Home
Page
|
|
IUPAC Prize for Young
Chemists - 2001
Honorable Mention
Current address (at the time of application)
University of North Carolina at Chapel Hill
Venable and Kenan Laboratories CB#3290
Chapel Hill, NC 27599-3290, USA
Tel: +1 919 962-0363
Fax: +1 919 962-2476
E-mail: [email protected]
Academic degrees
-
Dr. rer. nat. (Ph.D.) in Chemistry; Technische Universit�t
M�nchen, Germany; May 2000.
-
Diplom in Chemistry; Philipps-Universit�t Marburg,
Germany; September 1997.
-
Vordiplom in Chemistry; Johannes Gutenberg-Universit�t
Mainz, Germany; March 1994.
Ph.D. Thesis
Title Catalytic Activation of Aryl Chlorides
in Heck-Type Reactions
Adviser Prof. Dr. Dr. h.c. mult. Wolfgang A. Herrmann
Thesis Committee Ivar Ugi, Institut f�r Organische Chemie und
Biochemie, Technische Universit�t M�nchen; Wolfgang Beck, Lehrstuhl
f�r Anorganische Chemie, Ludwig-Maximilians Universit�t M�nchen; Klaus
K�hler, Institut f�r Anorganische Chemie, Technische Universit�t M�nchen.
Essay
Catalysis is of crucial importance for the development
of environmentally benign synthetic processes. Especially homogeneous
catalysis which combines aspects of organometallic chemistry and organic
synthesis allows highly selective transformations at mild conditions.
However, one of the major problems in homogeneous catalysis is the
activation of strong carbon-heteroatom bonds in cheap and abundant
starting materials. Therefore, the utilization of chloroarenes in
the palladium-catalyzed Mizoroki-Heck vinylation (1) and related cross-coupling
reactions (2) has been focused by catalyst development in recent years
both from synthetic and fundamental points of view.[1]
At the beginning of this Ph.D. thesis, only rare examples
of mostly activated chloroarenes were known to be reactive in transformations
of type (1) or (2).[2] Broad application
was hampered by either low stability of the catalyst or the demand
of high catalyst loading. In order to develop catalyst systems which
are capable of efficiently activating all types of chloroarenes at
low catalyst loadings, three different approaches were used:
1) For the optimization of catalyst systems, the understanding
of the reaction mechanism is of fundamental importance. As phospha-palladacycle
1 is an example for a highly active catalyst with bromoarenes,
the Mizoroki-Heck vinylation (1) was carefully examined in order to
determine the nature of the active species as well as the influence
of salt additives.
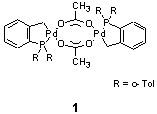
The interest in catalyst 1 was also driven by
a discussion in the literature about a possible, non-classical mechanism
involving Palladium(IV)-intermediates. Data supporting and disproving
this assumption had been presented in cause. In this Ph.D. thesis,
hydrogen isotope effects, Hammett correlations and product distributions
of catalyst 1 were compared to those of proposed intermediates
in a potential classical mechanism. This revealed that the mechanism
in case of catalyst 1 is related to classical proposals and
that Palladium(IV)-intermediates are very unlikely.
Furthermore, the kinetic studies reveal that salt additives
are responsible for a strong "special salt effect" induced by the
anion and a moderate "normal salt effect" due to the increased polarity
of the reaction medium. As a consequence, the reaction is best performed
in salt melts, i.e. ionic liquids. The optimized reaction medium [NBu4]Br
allows 1 and other frequently used catalysts, including highly
desirable but least active ligand-free palladium salts like PdCl2,
to perform substantially better. PdCl2 was previously only
known to activate iodoarenes and can now be used to couple bromo and
chloroarenes.
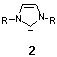
2) The first step in the catalytic cycles of the reactions
(1) and (2) is the oxidative addition to palladium(0) complexes. Due
to the reluctant activity of chloroarenes, we wanted to activate the
catalysts for this reaction step. Lewis-basic, electron-donating ligands
are known to promote oxidative additions.[3]
A novel class of electron-donating ligands are N-heterocyclic
carbenes 2 which have the advantage of forming extraordinarily
strong metal-ligand bonds allowing the formation of highly defined
catalyst structures including stable and recyclable immobilized catalysts
as was shown in this thesis.
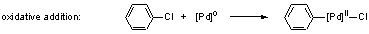
The first general synthetic route to homoleptic palladium(0)
complexes bearing N-heterocyclic carbenes was developed within
this thesis (reaction 3). In the Suzuki-Miyaura reaction (2), [M]
= B(OH)2, the most active catalyst of this type achieves
turnover-numbers and turnover-frequencies much higher than the best
published phosphine-system.
By transfer of knowledge gained with palladium as the
catalyst metal, highly active, homoleptic nickel(0) complexes of N-heterocyclic
carbenes were developed which are extraordinarily active in the Kumada
cross coupling reaction (2), [M] = MgCl. With the optimized catalysts,
even the selective activation of fluoroarenes, which were until
then considered to be unreactive in this type of reaction, could be
transformed.
3) Combinatorial catalysis is a convenient means of
screening a large amount of potential catalysts for their activity
in a desired reaction. As an essential necessity for efficient screening,
high-throughput must be achieved for (i) the synthesis of potential
catalysts, (ii) the desired reaction under inert atmosphere, and (iii)
the analysis of the reaction products.
For the first time, this thesis shows the use of N-heterocyclic
carbenes 2 as ligands in screening assays to be possible. A
modified synthesis route (4) of imidazolium salts as precursors to
the N-heterocyclic carbenes was developed which allows the
easy preparation of a large library of potential ligands. Taking advantage
of the in situ formation of N-heterocyclic carbenes
from imidazolium salts in the presence of a base, this library was
combined with transition metal salts. New reaction vessels were designed
to run the reactions under inert gas applying standard Schlenk-techniques.
Novel screening assays based on 19F-NMR and color identification
of the reaction products were developed and applied for the first
time.
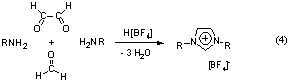
Highly active in situ catalyst systems consisting
of an imidazolium and a nickel or palladium salt were discovered by
these screening methods e.g. for the Kumada and the Negishi cross
coupling reactions (2), [M] = MgCl, ZnCl. In comparison to the defined
catalysts described above, these systems have the advantage of their
components being easy to prepare and being less sensitive towards
air and moisture.
In summary, within this thesis the activation of chloroarenes
(and in one particular example even fluoroarenes) in Heck-type
reactions (1) and (2) was achieved by novel catalysts and optimized
reaction conditions: (i) Studying mechanistic details led to the optimization
of the reaction conditions for the Mizoroki-Heck vinylation (1), (ii)
the application of novel ligands favoring the first step in the catalytic
cycles led to the discovery of novel, highly active catalysts for
the reactions (2), and (iii) in situ systems, which are especially
useful for organic synthesis in terms of handling, stability of the
components as well as convenience of preparation, were optimized by
high-throughput screening methods. In this thesis, no "magic" catalyst
for all transformations was identified but different structures had
to be optimized for each individual reaction owing to subtle differences
in the rate determining steps of the catalytic cycles. Thus, both
palladium and nickel complexes as well as phosphines and N-heterocyclic
carbenes 2 as ligands were found to be most active in different
reactions.
References:
[1] Applied Homogeneous Catalysis with Organometallic Compounds
(Eds.: B. Cornils, W. A. Herrmann), Wiley-VCH, Weinheim, 1996.
[2] see references in: (a) T. H. Riermeier, A. Zapf, M. Beller, Top.
Catal. 1998, 4, 301-309; (b) R. St�rmer, Angew. Chem.
Int. Ed. 1999, 38, 3307-3308.
[3] V. V. Grushin, H. Alper, Chem. Rev. 1994, 94, 1047-1062.
Page last modified 23 April 2001.
Copyright ©2001 International Union of Pure and Applied Chemistry.
Questions or comments about IUPAC, please contact, the Secretariat.
Questions regarding the website, please contact Web
Help.
|
| |
|
|