|
News
& Notices
Organizations & People
Standing
Committees
Divisions
Projects
Reports
Publications
Symposia
AMP
Links
of Interest
Search
the Site
Home
Page
|
|
Provisional Recommendations
Physical and Biophysical Chemistry
Division
Analytical Chemistry Division
Working Party on the Definition of pH Scales
Measurement of pH.
Definition, standards, and procedures
pH, defined by pH = - lg aH, is a single ion quantity;
hence, it is immeasurable unless evaluated by an associated convention.
Nevertheless, pH can be incorporated within the SI system of measurements,
because primary and secondary standards can be defined and determined
by the primary method or by secondary methods. Furthermore, incorporation
of the uncertainties for the primary method, and for all subsequent
measurements, permits the uncertainties for all procedures to be linked
to the primary standards by an unbroken chain of comparisons. Thus,
a rational choice can be made by the analyst of the appropriate procedure
to adopt to achieve the target uncertainty of sample pH.
Accordingly, this new document gives the IUPAC recommended definitions,
procedures, and terminology relating to pH measurements in dilute aqueous
solutions (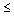 0.1
mol kg-1) in the temperature range 0-50 oC. 0.1
mol kg-1) in the temperature range 0-50 oC.
- The primary method is the cell without transference, called the Harned
Cell.
- The procedure for assigning pH(PS) values to primary buffer solutions
is based on the Bates-Guggenheim convention.
- The seven primary buffer solutions, to which pH(PS) values can be
assigned by the primary method, fulfill the required condition of "highest
metrological" quality, and are as follows:
- potassium hydrogen tartrate (saturated at 25 oC),
0.0341 mol kg-1
- potassium dihydrogen citrate, 0.05 mol kg-1
- potassium hydrogen phthalate, 0.05 mol kg-1
- disodium hydrogen orthophosphate, 0.025 mol kg-1 +
potassium dihydrogen orthophosphate, 0.025 mol kg-1
- disodium hydrogen orthophosphate, 0.03043 mol kg-1
+ potassium dihydrogen orthophosphate, 0.00869 mol kg-1
- disodium tetraborate, 0.01 mol kg-1
- sodium hydrogen carbonate, 0.025 mol kg-1 + sodium
carbonate, 0.025 mol kg-1.
- As there can be significant variations in the purity of samples of
a buffer substance of the same nominal chemical composition, the primary
buffer material used must be certified with values that have been measured
with the Harned cell.
- The certified pH(PS) values have target expanded uncertainties U
= 0.004, with a repeatability of 0.0015, reproducibility of 0.003, and
variations between batches of 0.003.
- To these uncertainties, a further contribution of 0.01, the assigned
uncertainty of the Bates-Guggenheim convention, must be added in order
to make a pH value traceable to the internationally-accepted SI system
of measurement.
- The pH(SS) values of secondary standard buffer solutions can be assigned
by the following procedures:
(i) Those substances that do not fulfill all the criteria for primary
standards but to which pH values can be assigned using the primary
method are considered to be secondary standards (target uncertainty
U = 0.01).
(ii) Secondary standards are also derived by secondary methods, which
allow the assignment of pH(SS) values by comparison with primary standards,
either of the same nominal composition or of different composition
(target uncertainties range from 0.004 to 0.015).
(iii) When secondary standards are not compatible with the use of
the platinum hydrogen electrode, the glass electrode can be used in
secondary methods (target uncertainty U = 0.02).
- Secondary methods use cells with transference which involve a liquid
junction potential contribution to the measured potential difference;
hence, the measurement equations contain terms that, although small,
are not quantifiable so that these methods are secondary, not primary,
methods.
- The target uncertainties for measuring the pH of unknown solutions
depend on the uncertainties of the standard buffer solutions and on
the chosen calibration procedure: multipoint (5-point), bracketing (2-point),
and 1-point + assumed practical slope calibration.
Details are given of the primary and secondary methods for measuring
pH and of the rationale for the assignment of pH values with appropriate
uncertainties to selected primary and secondary methods and to unknowns.
The present approach supersedes the previous compromise IUPAC Recommendations
[A.K. Covington, R.G. Bates, and R.A Durst, Pure Appl. Chem.
57, 531 (1985)], in that the focus is shifted from standard solutions
to standard methods and their associated uncertainties.
The unifying principle follows the simple path:
method + quality of materials 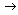 standard with known uncertainty
standard with known uncertainty
standard + calibration procedure 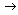 measurement of unknown pH with stated uncertainty
measurement of unknown pH with stated uncertainty
This approach requires the evaluation of the uncertainty associated
with each step in the process, starting from the assignment of pH(PS)
to a primary standard solution down to pH measurements of unknowns,
and to tracing the resulting combined uncertainty back to the primary
standards. Examples of uncertainty budget calculations, given in the
Annex, allow identification of the principal experimental parameters
affecting the accuracy of the selected procedure (to the target uncertainty
level), thus directing attention to the major error sources, and enabling
the minor ones to be disregarded.
> Download full text of the Provisional
Recommendations (pdf file - 180 KB)
Comments by 31 January 2002
Prof. Sandra Rondinini
Dipartimento di Chimica Fisica ed Elettrochimica
Universit� di Milano
Via Golgi 19
I-20133 Milano, Italy
Tel.: +39-02-26603-217
Fax: +39-02-26603-203
E-mail: [email protected]
> Project Description
Page last modified 23 July 2001.
Copyright ©2001 International Union of Pure and Applied Chemistry.
Questions or comments about IUPAC, please contact, the Secretariat.
Questions regarding the website, please contact Web
Help.
|

