|
News
& Notices
Organizations & People
Standing
Committees
Divisions
Projects
Reports
..By
Year
..Divisions
..Other Committees
..Provisional
Publications
Symposia
AMP
Links
of Interest
Search
the Site
Home
Page
|
Abbreviated
list of quantities, units and symbols in physical chemistry
4.7 physical quantities: thermodynamics
and statistics
| Physical Quantity |
Symbol |
SI Unit |
| heat |
q, Q |
J |
| work |
w, W |
J |
| thermodynamic temperature |
T |
K |
| Celsius Temperature |
q, t |
oC |
| internal energy |
U |
J |
| enthalpy |
H |
J |
| standard reaction enthalpy |
D r
H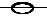
|
J mol-1 |
| entropy |
S |
J K-1 |
| Gibbs energy |
G |
J |
| Helmholtz energy |
A |
J |
| heat capacity |
C |
J K-1 |
| ratio Cp / Cv |
g, k |
1 |
| Joule-Thomson coefficient |
µ, µJT |
K Pa-1 |
| isothermal compressibility |
k |
Pa-1 |
| cubic expansion coefficient |
a |
K-1 |
| chemical potential |
µ |
J mol-1 |
| affinity of a reaction |
A |
J mol-1 |
| fugacity |
f, 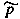 |
Pa |
| (relative) activity |
a |
1 |
| activity coeficient: |
| -- mixtures |
f |
1 |
| -- solutes |
g |
1 |
| osmotic coefficient |
f |
1 |
| osmotic opressure |
P |
Pa |
| equilibrium constant |
K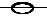 ,
K ,
K |
1 |
| -- on a concentration basis |
Kc |
(mol m-3)Sv |
| -- on a pressure basis |
Kp |
PaSv |
| -- on a molality basis |
Km |
(mol kg-1)Sv |
| density of (energy) states |
r(E) |
J-1 |
| statistical weight |
g |
1 |
| partition function: |
| -- particle |
q, z |
1 |
| -- canonical ensemble |
Q, Z |
1 |
| -- microcanonical ensemble |
W |
1 |
| symmetry number |
s, s |
1 |
| charateristic temperature |
Q |
K |
Page last modified 12 June 2000.
Copyright © 2000 International Union of Pure and Applied Chemistry.
Questions or comments about IUPAC, please contact, the Secretariat.
Questions regarding the website, please contact web
manager.
|

