|
|
Vol.
26 No. 4
July-August 2004
| Up for Discussion |
| |
A forum for members and member organizations to share ideas and concerns.
Send your comments by e-mail to [email protected] |
Questionable Stereoformulas of Diastereomers
The comments below have been received in response to the ideas of using "thick" and "hatched" bonds to represent relative configurations expressed by G. Kaupp and M. Reza Naimi-Jamal in their article in the Jan-Feb 2004 Chemistry International (in print page 15). These comments add to the debate and will be considered in the course of the recently initiated IUPAC project on Graphical Representation Standards for Chemical Structure Diagrams, chaired by William Town—see project announcement (in print page 23).
by H.D. Flack
Kaupp and Naimi-Jamal's comments and suggestions are to be taken very seriously for the reporting of crystal and molecular structures determined by X-ray diffraction. We take this opportunity to express our point of view on some matters that relate directly to this subject.
No
matter what graphical representation is finally agreed upon
for molecular diagrams, we consider it essential that these
must be usable by a human chemist or crystallographer and
allow for a high degree of automatic checking by a computer.
Editors, co-editors, and editorial staff do not have the time
to go around checking the stereochemistry and labelling of
every diagram in every paper. Automation provides a very cost-effective
aid in the process of verifying scientific communications.
The molecular diagram must be machine-interpretable. Molecular
diagrams, molecular identifiers, and molecular names must
be designed so that they are in one-to-one correspondence
with one another. Moreover, any caption labelling of a molecular
diagram must be compatible with the presented molecular diagram.
In terms of the reporting of molecular structures from crystal-structure
determinations, we distinguish three distinct cases: enantiopure
compound of known absolute configuration, enantiopure compound
of unknown absolute configuration, or racemate.
The “Basic Terminology of Stereochemistry,” (1996) Pure Appl. Chem. 68, 2193–2222, gives the terminology for attributing distinct names to the compounds in these three classes. However Kaupp and Naimi-Jamal’s suggestions for molecular diagrams do not produce graphical representations of enantiopure compound of unknown absolute configuration distinct from racemate, and conversely. However the physical state of these two classes is very different and such properties as the melting point, optical activity, and CD spectrum would be different. Although we have noted elsewhere [H.D. Flack (2003), Helv. Chem. Acta 86, 905–921] that the IUPAC naming of racemates may readily be extended to take account of enantiomeric mixtures in rational proportions other than 1:1, it is completely unclear to us how molecular diagrams should be presented in such cases.
The late André Collet brought to our attention that the definition of absolute configuration as given in the “Basic Terminology of Stereochemistry” (1996) is inadequate in one important respect. Essentially, his argument is that it is useless to specify a stereo-configuration by a molecular diagram or molecular name unless it is accompanied by an appropriate physical characterization of the bulk compound. [see H. D. Flack and G. Bernardinelli (1999) Acta Cryst. A55, 908–915 for a full and open-access discussion.]
H.D.
Flack <[email protected]>
is professor at the Laboratoire de Cristallographie, at the
University of Geneva, Switzerland. <www.flack.ch/howard/cristallo/Howard.Flack.html>
by Michinori Oki
As
someone interested in conformation, I cannot agree with Kaupp
and Naimi-Jamal's suggestion because it is quite different
from common practice.
Their
paper states that “wedged chemical formula should be
used only for indication of absolute configuration.”
We had known that IUPAC wanted to indicate stereochemistry
by using solid or hatched lines as shown in the figure below.
But this is very inconvenient in discussing conformations.
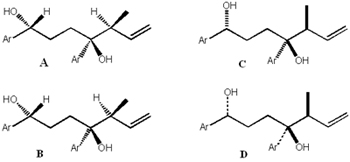 |
| Figure
reprinted from Jan-Feb 2004
CI. According to Kaupp and Naimi-Jamal, formula
A, or even more distinct formula B (traditionally with
exactly the same meaning), will then rightfully look like
formula C (alternatively like formula D), if it is not
the (1R,4S,5S)-enantiomer, but the racemate of this diastereomer. |
We
therefore have used wedges to indicate that the bond in question
is directed to the rear of the paper and the solid wedge directed
toward the eye. Incidentally, normal lines or solid or hatched
lines, if they do not change in width, mean that these are
in the plane of the paper. Therefore, the formula C below
does not provide any information about conformation. However,
if one use wedges, it is easy to recognize that a bond in
question is is antiperiplanar (ap) or synclinal (sc) to a
given bond that is attached to the neighboring carbon. I hope
that IUPAC does not adopt Kraupp’s proposal, unless
the usage of formula C is confined to indication of configurations.
Rather, wedges should be recommended for indication of the
direction of a bond in question.
Michinori Oki <[email protected]> is an IUPAC Fellow who recently retired from the Okayama University of Science, Japan.
Link
to Kaupp and Naimi-Jamal’s original article:
www.iupac.org/publications/ci/2004/2601/ud1.html
Page
last modified 18 July 2004.
Copyright © 2003-2004 International Union of Pure and
Applied Chemistry.
Questions regarding the website, please contact [email protected]
|