> Risk Assessment
Terminology
> Medicinal Chemistry
> News and Notices
> Project Reports
> Symposia Reports
> New Projects
> Provisional Recommendations
> New Books
> Commissions Reports
> Conference
Announcements
> Conference Calendar
Chemistry International
Vol. 23, No. 2
March 2001
Risk Assessment Terminology

This article is a summary of the main points of a report of the work of a joint International Program on Chemical Safety/Organization for Economic Cooperation and Development (IPCS/OECD) Working Party that was published in 1999 in Terminology Standardization and Harmonization (see ref 1).
The problem of "risk" and "hazard" terminology has bedeviled discussions relating to the safe use of chemicals, because there have been different usages, although a consensus is emerging. This paper describes that consensus and how the Working Party identified it. We believe that the chemical community should be aware of the consensus position, and we think that the methodology applied is of considerable interest and agrees with that used by IUPAC terminologists.
Recently, because of my IUPAC experience in compiling the "Glossary for Chemists of Terms Used in Toxicology", I was invited to take part in a project with the objective of harmonizing the chemical hazard/risk assessment terminology used by risk assessors. The project was established under the auspices of the International Program for the Good Management of Chemicals (IOMC), with the active involvement of the International Program on Chemical Safety (IPCS) and the Organization for Economic Cooperation and Development (OECD). Experts on chemical risk assessment from a variety of backgrounds, including chemistry, biochemistry, pharmaceuticals, toxicology, food safety, environmental sciences, and epidemiology, contributed to the project. Most importantly, the core working group included specialists in terminology, and this composition greatly facilitated its procedures. A full account of the project with detailed annotation has recently been published [2]. This paper presents an outline of the process adopted to identify consensus definitions of the relevant terms and lists the definitions in their current forms. For a thorough discussion, see the full published account [2].
A joint terminology steering committee (TSC) was established with a balanced representation from the parent organizations to provide guidance and validate proposed approaches ex ante. In particular, each member of the TSC had to define the nature and magnitude of the terminological difficulties encountered by their constituency.
A smaller terminology planning group (TPG) was established to propose solutions and, once proposals were endorsed, to proceed accordingly. The work plan was organized in five phases.
1. Preliminary work. The members of the TSC were invited to identify, independently and individually:
a) terms that, in their opinion, should be covered by the project. Terms were eligible for inclusion in the project when differences in usage in various groups were perceived to hamper interdisciplinary cooperation. For practical reasons, it was agreed to limit the initial list to 50 terms;
b) reference materials, such as glossaries, dictionaries, and other documents which, in their individual opinion, were the most reliable for understanding the meaning of the selected key terms;
c) peers who would constitute an international reference group (IRG) and participate in a large consultation that would inform the harmonization process.
2. Priming the process. Materials received from the TSC members were preprocessed as follows:
a) An initial cumulative list of more of than the 200 terms was compiled and circulated iteratively to the terminology steering committee for reduction to the agreed level of 50 terms;
b) All reference materials were screened and all definitions entered in a database for future reference. The database eventually totaled some 5 000 terms and 15 000 definitions;
c) A survey document was prepared listing the 50 selected key terms in alphabetical order, together with the corresponding definitions collected from the reference materials; the number of definitions per term ranged from 1 to 23, giving a total of 350 in all.
3. Launching the survey. A consensus-building exercise was initiated among the 200 peer-nominated experts constituting the IRG. Participants in the survey were invited to indicate, for each of the selected terms, the definition they preferred. Only one choice was permitted. Comments were invited, where necessary, to refine one’s opinion on the selected preferred definition. A none-of-the-above option was added to permit rejection of any of the listed definitions; in such a case, comments were mandatory. Survey forms were sent on paper to the participants and made available through the Internet.
4. Analyzing the data. Ten thousand records were received, stored in a database, and tabulated. Preferences were counted, and comments were studied. Possible interdisciplinary biases were checked. A detailed report was prepared, giving a snapshot picture of the terminology understanding that emerged from the survey.
5. Review by the terminology planning group. A critical review was conducted to assess the conformity of the results arrived at during the analytical process. The outcome of the project, the final list of definitions adopted, is given below.
The terms are not listed here in alphabetical order, because we were dealing with concepts and it was found best to consider each term in the appropriate conceptual environment. We called the base terms "data-oriented terms" and their combinations with action concepts "action-oriented terms".
1. Data-oriented terms. "Risk" and "hazard" are the key data-oriented terms, and there are clusters of related terms around them. Others include "guidance value", "margin of exposure", "safety factor", and "threshold".
2. Action-oriented terms. These are terms used in conjunction with single-word terms, except for "assessment", which is defined in isolation also.
Online feature: alternative alphabetical index Note: For the purpose of this terminology, the term "agent" refers to any chemical, physical, or biological entity.
SYMBOLS USED None
term from the original list of terms, considered to be a base term term added to the initial list of terms for reasons of consistency
term intimately related to the preceding base term term loosely related to the preceding base term
HAZARD vs. RISKhazard: inherent property of an agent or situation capable of having adverse effects on something. Hence, the substance, agent, source of energy, or situation having that property
risk: the probability of adverse effects caused under specified circumstances by an agent in an organism, a population, or an ecological system
DOSE vs. CONCENTRATION
dose: total amount of a substance administered to, taken, or absorbed by an organism
concentration: quantity of a material or substance contained in unit quantity of a given medium or system
EFFECT vs. RESPONSE
effect: change in the state or dynamics of a system caused by the action of an agent
response: change developed in the state or dynamics of a system in reaction to the action of an agent
adverse effect: change in morphology, physiology, growth, development, or life span of an organism, which results in an impairment of functional capacity, an impairment of the capacity to compensate for additional stress, or an increase in susceptibility to other environmental influences
dose-related effect: change to a system as a function of the quantity of a substance administered, taken, or absorbed by it
dose-effect relationship: link between the total amount of a substance administered, taken, or absorbed by a system and the magnitude of a specific, continuously graded change affecting it
Related term: effect assessment below
concentration-effect relationship: link between the exposure of a given system to a substance over time and the magnitude of a specific, continuously graded change to that system
exposure: Concentration, amount, or intensity of a particular agent that reaches a target system. It is usually expressed in numerical terms of substance concentration, duration, frequency, and intensity (after ref. 3)
dose-response relationship: link between the amount of an agent absorbed by a population and the change developed in that population in reaction to it
Note: It may be expressed as the proportion of a population exposed to an agent that shows a specific reaction. It may also be used to signify the magnitude of an effect in one organism (or part of an organism); in that case, it is more specifically called "dose-effect relationship".
dose-response curve: graphical presentation of a dose-response relationship
SAFETY AND UNCERTAINTY
safety: practical certainty that adverse effects will not be caused by an agent under defined circumstances
Note: It is a reciprocal of risk.
safety factor: factor by which an observed or estimated toxic concentration or dose is divided to arrive at a criterion or standard that is considered safe
margin of exposure: ratio of the no-observed-adverse- effect level (NOAEL) to the estimated exposure dose (EED) or concentration (EEC)
Note: In the case of environmental risk assessment, predicted environmental concentration (PEC) is used instead of EEC.uncertainty: imperfect knowledge concerning the present or future state of a system under consideration
ACCEPTABLE DAILY INTAKE
acceptable daily intake: maximum amount of a substance to which a subject may be exposed daily over the subject’s lifetime without appreciable health risk
tolerable intake: estimate of the amount of a substance that can be ingested or absorbed over a specified period of time without appreciable health risk
MISCELLANEOUS
guidance value: value, such as concentration in air or water, that is derived after appropriate allocation of the reference dose among the possible media of exposure to assist regulatory authorities in establishing permissible levels of a potential toxicant
Note: "Reference dose" is a term used in the United States for an estimate (with uncertainty spanning perhaps an order of magnitude) of daily exposure to the human population (including sensitive sub-groups) that is likely to be without appreciable risk of deleterious effects during a lifetime [4].threshold: dose of a substance or exposure concentration below which a stated effect is not observed or expected to occur
expert judgment: opinion of an authoritative person on a particular subject
toxicity: inherent property of a substance to cause an adverse biological effect
validation: process of assessing whether the predictions or conclusions reached in a risk assessment are correct
ASSESSMENT VS. ANALYSIS
assessment: combination of analysis of facts and inference of possible consequences concerning a particular object
assessment endpoint: quantitative expression of a specific factor with which a risk may be associated as determined through an appropriate risk assessment
assessment factor: numerical adjustment used to extrapolate from experimentally determined dose- response relationships to estimate the substance exposure at and above which adverse effects may occur
analysis: detailed examination of anything complex made in order to understand its nature or to determine its essential features
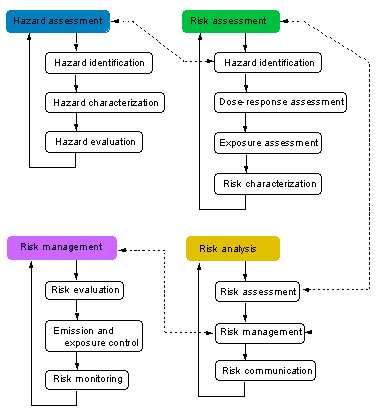
HAZARD ASSESSMENT [back to chart]
hazard assessment: process designed to determine factors contributing to the possible adverse effects of a substance to which a human population or an environmental compartment could be exposed. The process includes three steps: hazard identification, hazard characterization, and hazard evaluation (see Fig. 1).
Note: Factors may include mechanisms of toxicity, dose-effect and dose-response relationships, variations in target susceptibility, etc.
hazard identification: [HAZARD ASSESSMENT] the first stage in hazard assessment, consisting of the determination of substances of concern, the adverse effects they may have inherently on target systems under certain conditions of exposure, taking into account toxicity data
Note: Definitions may vary in wording, depending on the context. Thus, here: [RISK ASSESSMENT] the first stage in risk assessment, consisting of the determination of particular hazards a given target system may be exposed to, including attendant toxicity data.
hazard characterization: the second step in the process of hazard assessment, consisting in the qualitative and, wherever possible, quantitative description of the nature of the hazard associated with a biological, chemical, or physical agent, based on one or more elements, such as mechanisms of action involved, biological extrapolation, dose-response and dose-effect relationships, and their respective attendant uncertainties
hazard evaluation: the third step in the process of hazard assessment aiming at the determination of the qualitative and quantitative relationship between exposure to a hazard under certain conditions, including attendant uncertainties and the resultant adverse effect
RISK ASSESSMENT [back to chart]
risk assessment: process intended to calculate or estimate the risk for a given target system following exposure to a particular substance, taking into account the inherent characteristics of a substance of concern as well as the characteristics of the specific target system. The process includes four steps: hazard identification, dose-response assessment, exposure assessment, and risk characterization. It is also the first step in risk analysis.
hazard identification: [RISK ASSESSMENT] the first stage in risk assessment, consisting of the determination of particular hazards a given target system may be exposed to, including attendant toxicity data
Note: Definition may vary depending on the context. Thus, here: [HAZARD ASSESSMENT] the first stage in hazard assessment, consisting of the determination of substances of concern and the adverse effects they may inherently have on target systems under certain conditions of exposure, taking into account toxicity data.
dose-response assessment: the second of four steps in risk assessment, consisting of the analysis of the relationship between the total amount of an agent absorbed by a group of organisms and the changes developed in the group in reaction to the agent, and inferences derived from such an analysis with respect to the entire population
effect assessment: combination of analysis and inference of possible consequences of the exposure to a particular substance based on knowledge of the dose-effect relationship associated with it in a specific target system
exposure assessment: [RISK ASSESSMENT] step in the process of risk assessment, consisting of a quantitative and qualitative analysis of the presence of an agent (including its derivatives) that may be present in a given environment and the inference of the possible consequences it may have for a given population of particular concern
Note 1: [engineering] determination, through the use of a variety of analytical techniques, of the quantity and fate of a chemical, physical, or biological agent in a medium of concern.
[hazard assessment] process to analyze, using a range of different techniques, the amount of a chemical, physical, or biological agent that could be present in a given medium and the fate of such agent under a number of potential circumstances, and to infer possible consequences for a hypothetical system that could be affected by it.
Note 2: Exposure assessment may imply taking into account duration, frequency, or concentration, including considerations of bioavailability.
exposure scenario: set of conditions or assumptions about sources, exposure pathways, concentrations of toxic chemicals, and populations (numbers, characteristics, and habits) that aid the investigator in evaluating and quantifying exposure in a given situation [5]
or
set of assumptions concerning how an exposure may take place, including assumptions about the exposure setting, stressor characteristics, and activities that may lead to exposure [1]
fate: pattern of distribution of a substance, its derivatives, or metabolites in a system of concern as a result of transport, partitioning, transformation, or degradation
risk characterization: integration of evidence, reasoning, and conclusions collected in hazard identification, dose-response assessment, and exposure assessment and the estimation of the probability, including attendant uncertainties, of occurrence of an adverse effect if an agent is administered, taken, or absorbed by a particular organism or population. It is the last step of risk assessment.
Note: In ecological risk assessment, concentration-response assessment is carried out instead of dose-response assessment.
or
qualitative and/or quantitative estimation, including attendant uncertainties, of the severity and probability of occurrence of known and potential adverse effects of a substance in a given population
risk estimation: quantification of the probability, including attendant uncertainties, that a chemical, physical, or biological agent administered, taken, or absorbed by a system with have a specific effect, based on hazard identification, dose-response assessment, and exposure assessment for that particular agent in relation to that particular system
acceptable risk: type of risk such that the benefits derived by an organism, a population, or an ecological system outweigh the adverse effects that might affect them as a result of being administered or exposed to a particular agent
RISK MANAGEMENT [back to chart]
risk management: decision-making process involving considerations of political, social, economic, and technical factors with relevant risk assessment information relating to a hazard so as to develop, analyze, and compare regulatory and nonregulatory options and to select and implement the optimal decisions and actions for safety from that hazard. Essentially, risk management is the combination of three steps: risk evaluation, emission and exposure control, and risk monitoring.
Note: The intermediate step (emission and exposure control) was not listed in the survey, but is included here for the sake of consistency. Control is used here in a general rather than specific regulatory sense.
risk evaluation: establishment of a qualitative or quantitative relationship between risks and benefits, involving the complex process of determining the significance of the identified hazards and estimated risks to those organisms or people concerned with or affected by them. It is the first step in risk management.
Note: It is synonymous with risk-benefit evaluation.
risk monitoring: process of following up the decisions and actions within risk management in order to ascertain that risk containment or reduction with respect to a particular hazard is assured
RISK ANALYSIS [back to chart]
risk analysis: process for controlling situations where populations or ecological systems could be exposed to a hazard. It usually comprises three steps, namely risk assessment, risk management, and risk communication.
risk assessment: process intended to calculate or estimate the risk for a given target system to be affected by a particular substance, taking into account the inherent characteristics of the substance of concern as well as the characteristics of the specific target system. The process includes four steps: hazard identification, dose-response assessment, exposure assessment, and risk characterization.
risk management: decision-making process involving considerations of political, social, economic, and technical factors with relevant risk assessment information relating to a hazard so as to develop, analyze, and compare regulatory and nonregulatory options, and to select and implement the optimal response for safety from that hazard. Essentially, risk management is the combination of three steps: risk evaluation, emission and exposure control, and risk monitoring.
Note: The intermediate step (emission and exposure control) was not listed in the survey, but is included here for the sake of consistency.
risk communication: interactive exchange of information about risks among risk assessors, managers, news media, interested groups, and the general public
1. P. Lewalle. "Risk Assessment Terminology: Methodological Considerations and Provisional Results". Terminology Standardization and Harmonization 11 (1-4), 1-28. (1999).
2. D. G. Barnes and M. Dourson. Regul. Toxicol. Pharmacol. 8, 471-486 (1988).
3. J. H. Duffus. "Glossary for chemists of terms used in toxicology", Pure Appl. Chem. 65 (9), 2003-2122 (1993).
4. IRIS. Glossary of Risk Assessment Related Terms, U.S. Environmental Protection Agency, Washington, DC (1992).
IRIS online <http://www.epa.gov/ngispgm3/iris/>
5. Council on Environmental Quality. Risk Analysis: A Guide to Principles and Methods for Analyzing Health and Environmental Risks. PB89-137772. National Technical Information Service, Washington, DC (1989).** Dr. John H. Duffus (Director, Edinburgh Centre for Toxicology, 43 Mansionhouse Road, Edinburgh, EH9 2JD, Scotland, UK; E-mail: [email protected]), Chairman of the IUPAC Commission on Toxicology (VII.C.2), submitted the article that appears above.
News
and Notices - Organizations and People
- Standing Committees
Divisions
- Projects - Reports
- Publications - Symposia
- AMP - Links
Page last modified 23 February 2001.
Copyright © 1997-2001 International Union of Pure and Applied Chemistry.
Questions or comments about IUPAC, please
contact the Secretariat.
Questions regarding the website, please contact [email protected]